
1)
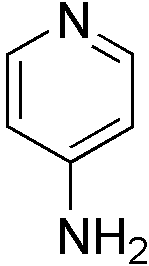
4-Aminopyridine
2) HP-184
3)
Theophylline
4) Lithium
5) Fibroblast Growth Factor
1)
4-Aminopyridine (4AP or Fampridine-SR): 4AP is a small molecule
that restores some function in some individuals with SCI. Basically, it
works by improving the conduction of intact but demyelinated neurons that
often still transverse the SCI injury site in not only incomplete but
clinically classified complete injuries. It has been estimated that a
third of those with SCI can accrue some benefit from 4AP.
Because demyelinated neurons have lost their
insulating sheath, they no longer can transmit a signal. Without the
insulating myelin, potassium channels (called fast voltage-sensitive
potassium channels) are exposed on the axonal surface, letting potassium
ions leak out of the axon, in turn, short-circuiting neuronal
transmission. By blocking these channels, 4AP increases action potential
duration, allowing signal conduction through the demyelinated sections
and, as a result, increasing neurotransmitter release from the axon.
Because its mechanism of action involves demyelinated
neurons, 4AP was first tested in individuals with multiple sclerosis (MS).
Both SCI and MS are disorders characterized by the loss of insulating
myelin around neurons; in SCI, the loss is from injury, and with MS, it is
from autoimmune-mediated causes.
Because 4AP does not stimulate regeneration or
improve any post-injury physiology or biology, it not a cure but a
transient enhancer of existing function, i.e., a temporary fix.
Once the body eliminates 4AP, benefits are lost. The
sustained-release formulation of 4AP (Fampridine-SR) peaks in the blood
about 2-3 hours after administration and has a half life of roughly 6-7
hours. In studies measuring the
elimination of orally administered 4AP tagged with a radioactive tracer,
virtually all of the drug had been eliminated 24 hours after
administration. To maintain a benefit-sustaining plasma level, 4AP must be taken
several times daily on an ongoing basis. In other words, any restored
function will dissipate after stopping the drug.
Surveys taken on SCI-focused websites suggest that
15% of those with SCI have taken 4AP, which is available through
compounding pharmacies (i.e., pharmacies that make medicines from agents
that doctors prescribe). The recommended daily dose of the
immediate-release formulation made by the compounding pharmacies is 40
mg/day, administered in periodic 10-mg doses. In contrast, Acorda
Therapeutics has developed a sustained-released formulation (Fampridine-SR)
with a longer half life. With this formulation, a 40-mg dose can be given
twice a day.
Originally used as a bird repellent (trade name
Avitrol), 4AP is not innocuous and can have significant side effects.
Finding a dose that maximizes benefits while minimizing side effects has
been challenging. In some, the most effective dose is close to one that
causes side effects. Because 4AP stimulates the nervous system, side
effects include insomnia, nervousness, tingling, increased blood pressure,
heart rate, and seizures.
In a case report, 4AP’s potential toxicity was
documented in a 42-year-old man with a C3 injury, who had been taking
4AP prepared by a compounding pharmacy (ref). His 4AP pills contained
10-times the dose indicated on the label, a dose which routinely causes
toxicity. As a result, he had to be taken to the hospital emergency
room. According to the report, “He recovered with permanent short-term
memory loss after a prolonged and complicated hospital course.”
There seems to be some dichotomy between patients and
scientists on 4AP’s risks relative to benefits. Specifically, many
professional articles seem to minimize the risks while much of the
anecdotal discussion posted on SCI websites suggests benefits are subtle
relative to side effects.
Acorda Therapeutics, a biotechnology company focused
on spinal cord dysfunction, has been the leading force behind the
development of 4AP as a function-restoring agent for SCI. In fact, many of
the published papers have been authored by scientists who have close
associations with Acorda.
Unfortunately, after a decade of commitment, Acorda
4AP’s research-and-development efforts have fallen short for SCI, and the
company is redirecting it efforts toward the substance’s MS benefits.
Specifically, in 2004, the company completed two
phase-3 clinical trials involving their sustained-release formula
Fampridine-SR. Perhaps due to a number of confounding issues that lessened
study robustness, trial results did not demonstrate sufficient statistical
significance for the two endpoints measured, spasticity reduction (using
the Ashworth score) and the Subject Global Impression rating. Acorda’s
problems underscore the overall difficulty of selecting appropriate test
endpoints for potential function-restoring interventions for chronic SCI.
Considerable animal and human research has been
carried out on 4AP over the last several decades. Some of the early animal
research leading up to human trials were succinctly reviewed by Dr.
Stephen Waxman (New Haven, Conn) (J Neurotrauma 10(1), 1993).
The following summarizes in chronological order some
key human studies:
1) Hansebout et al. (Hamilton, Ontario) administered
4AP by infusion to eight subjects with chronic SCI using a randomized,
crossover design, in which each patient received 4AP or placebo and then
switches treatment. Improvements were noted in five of six subjects with
incomplete SCI; none were observed in the two patients with complete
injuries. Improvements included increased motor control and sensitivity,
and reduction in pain and spasticity (J Neurotrauma 10(1), 1993).
2) Hayes and colleagues (London, Ontario) assessed
the effects of intravenous 4AP on electrophysiological conduction in six
patients with SCI. Two patients had increases in amplitude of cortical
somatosensory evoked potentials (SEPs), and four had increases in
amplitude of motor- evoked potentials (MEPs). Clinical improvements
included reduced spasticity (2) and pain (1), and increased sensation (1),
leg movement (3), and bowel control (1) (J Neurotrauma 11(4),
1994).
3) Segal et al (Long Beach, California) demonstrated
that a single 10-mg dose of 4AP improved pulmonary function in patients
with quadriplegia. Specifically improvements were noted in forced vital
capacity, maximal inspiratory and expiratory pressure beginning six hours
and lasting 12 hours after dosing (Pharmacotherapy 17(3), 1997).
4) Potter et al. (London, Ontario) described
improvements in three patients with incomplete quadriplegia who took 10-mg
oral doses of 4AP twice or three times daily over four months. In addition
to marked and sustained reduction in spasticity, other benefits included
reduced pain (1), restored muscle strength (3), improved sensation (2),
voluntary bowel control (1), erectile function (2), improved hand function
(1), and enhanced transfers and gait (2). One patient stood with support
for the first time since injury 16 years earlier (Spinal Cord
36(3), 1998).
5) Potter et al (London, Ontario) reported the
results of a randomized, double-blind, crossover study designed to assess
the safety and efficacy of the Fampridine-SR sustained-release
formulation. Twenty-six patients with incomplete SCI received either 4AP
or placebo for two weeks followed by a one one-week washout period after
which treatments were reversed. Improvements were reported with patient
satisfaction, quality of life, sensation, motor function, and spasticity.
No statistically significant benefits were documented for pain; bowel,
bladder, or sexual function; or functional independence (J Neurotrauma
15(10), 1998).
6) Segal et al (Long Beach, California) studied 4AP’s
effects on ambulatory parameters in nine males (6 quadriplegic and 3
paraplegic) with some walking ability. Parameters included velocity
(meters/min), cadence (steps/min), stride length (meters), gait cycle
(seconds), and double-limb support (percent of gait cycle). After 4AP
dosing, statistically significant changes were noted in various parameters
(J Spinal Cord Med 21(3), 1998).
7) In a randomized, dosage-blinded study, Segal et al
(Long Beach, California) compared sensorimotor function in 16 subjects
with SCI who had received 30-mg/day 4AP (high-dose) for three months with
five subjects who received a 6-mg/day low dose. High-dose patients had
decreased spasticity, and improved motor and sensory scores and pulmonary
function (Pharmacotherapy 19(6), 1999).
8) In a randomized, double-blind, crossover study,
Van der Bruggen et al (Amsterdam, Netherlands) concluded that 4AP had no
statistically significant effects on functional status, walking speed and
vibration perception in 20 patients with incomplete SCI (J Neurol
8(8), 2001).
9) Segal et al (Long Beach, California) compared the
effects of a single 10-mg, 4AP dose on heart-rate variability - as a
measure of the autonomic nervous system - in 13 subjects with SCI with 13
able-bodied controls. The difference in pre-test heart-rate variability
between subjects with SCI and able-bodied controls disappeared in the 24
hours immediately after 4AP administration (Am J Ther 9(1) 2002).
10) Grijalva and colleagues (Mexico City, Mexico)
randomized 27 patients with SCI to receive an escalating 4AP dose or
placebo for 12 weeks, after which treatments were reversed. Compared to
placebo controls, improved motor function, sensation and independence were
observed in 4AP-treated patients. The investigators noted persistent 4AP
effects on sensation and independence 12 weeks after subjects were
switched to placebo. Fifty-six percent of patients had adverse reactions (Pharmacotherapy
23(7), 2003).
11) Hayes and colleagues (London, Ontario) studied
pharmacokinetics in 16 patients with incomplete SCI (ASIA grade B-D) after
dosing with 25-60 mg of Fampridine–SR twice daily for one week. Peak
plasma concentration occurred 2.2-3.0 hours after dosing and drug half
life was 5.7-6.9 hours. The investigators concluded that 1) adverse
effects were mild or moderate and not dosage related and 2) this sustained
release formulation was slowly absorbed and eliminated (Arch Phys Med
Rehabil 85(1), 2004).
12) Acorda Therapeutics sponsored a phase-2
clinical trial comparing subjects treated with high- or low-dose,
sustained-released 4AP formulations (i.e., Fampridine SR) with
placebo-treated subjects (Cardenas DD, et al, Spinal Cord 45,
2007). Each group contained ~30 subjects. Subjects had sustained
incomplete injuries at least 18 months earlier (i.e., chronic injuries),
ranged in age from 18 to 70 years, and had injuries between the cervical
C4 and thoracic T10 levels. Eighty percent were men, and 92% were
Caucasian.
High and low-dose subjects received 40 and 25
milligrams of the drug, respectively, twice daily for four weeks.
Forty-three percent of the high-dose subjects dropped out mostly due to
a variety of adverse side effects. The low-dose regimen seemed to be
better tolerated with an attrition rate of only 13% compared to 10% for
placebo-treated subjects, respectively.
Outcomes were evaluated by a variety of
measurements, including, patient-diary questionnaires, quality-of-life
assessments, erectile function, bladder and bowel management,
spasticity, and clinician views of overall improvement. Although the
study lacked the statistical robustness to draw firm conclusions for
many of the outcome variables, some improvements seemed to accrue for
quality of life, spasticity, and erectile function.
13) Segal and colleagues (USA) evaluated
4AP’s effects on glucose tolerance in 31 individuals with injuries
sustained at least a year earlier (ref). In general, individuals with
SCI are prone to impaired glucose tolerance or diabetes. After fasting,
subjects ingested 75 grams of glucose and completed a five-hour
glucose-tolerance test, an assessment which basically measures how
effectively the body can clear glucose from the blood. Before treatment,
29 of the 31 subjects had impaired glucose tolerance. In contrast, after
six months of treatment with an oral, immediate-release 4AP formulation,
only 12 had impaired glucose tolerance.
14)
Grijalva and associates (Mexico)
evaluated the impact of 4AP on gastric emptying in patients with chronic
injuries (ref). SCI can adversely affect the gastrointestinal tract,
slowing the movement of solids though the stomach and colon. Signs of a
neurogenic bowel include fecal impaction, constipation, abdominal
distention, prolonged bowel care, and delayed colonic transit. It has
been estimated that 41-86% of individuals with SCI have some of these
problems, and 41% spend at least one hour daily on bowel care.
In this
specific study, 18 subjects with chronic SCI (9 with cervical injuries
and 9 with thoracic injuries) were given daily oral doses of 4AP for 12
weeks. Gastric emptying was measured before and after this period. The
results indicated that “4AP intake in patients with chronic spinal cord
injury significantly slowed gastric emptying regardless of level and
ASIA score of injury.”
15) In 2012,
Dr. Trevor Dyson-Hudson and
colleagues (USA) announced the initiation of a double-blind study to
evaluate the efficacy, safety and tolerability of 4AP treatment in
combination with locomotor training in individuals with motor-incomplete
injuries sustained at least 12 months before enrollment. The
investigators intend to recruit ~46 individuals between 18 and 70 years
old with injuries ranging from the cervical C4 to thoracic T10 level.
Subjects will be randomized to receive either 10-mg of extended release
tablets of 4AP (called dalfampridine) or placebo twice daily for 10
weeks. During this period, all subjects will undergo locomotor training
therapy five times weekly. The primary outcome will be walking ability,
which will be assessed at baseline, week 5, week 10, and 12 weeks after
treatment has stopped.

2) HP-184:
Like 4-AP discussed above, HP-184 enhances the conduction of
intact, but demyelinated, axons that often still traverse the injury site
in most injuries. As a conduction enhancer, it is not a cure in terms of
permanent, function-restoring neuronal regeneration, but a stimulator of
all-ready existing neurons that will wear off once the drug is metabolized
and eliminated from the body.
Basically, conduction depends on the sequential flow
of various ions in and out of neurons, a process that is disrupted when
the neuron loses its insulating sheath of myelin. HP-184 is a
potassium/sodium-channel blocker, which works, as the name implies, by
blocking conduction-inhibiting ion channels that are exposed after
injury-induced loss of myelin.
Sanofi-Aventis Pharmaceuticals has sponsored several
phase-2 (i.e., intermediate) clinical trials assessing HP-184’s safety and
efficacy. According to a NIH listing of clinical trials (www.clincialtrials.gov),
the most recent double-blind, multi-center study was initiated in 2004
with a targeted enrollment of 240 subjects with ASIA C-D incomplete (see
appendix) injuries sustained at least 18 months before study entry (i.e.,
chronic injuries). The injury level of enrolled subjects had to be between
C4 and T10.
Although few results have been reported, several
subjects have individually discussed their experiences. For example, after
six months of treatment, one patient indicated she had regained full body
sensation and considerable muscle strength. In another case, a woman
reported “I was standing strong. I was actually starting to do stairs.
Everything was kicking in…” Side effects included headaches, nausea, and
fatigue. As expected with a conduction-enhancing drug, all improvements
went away after discontinuation of the dosing regimen.

3)
Theophylline Stimulation of Respiration:
Evidence indicates that theophylline stimulates respiration in
individuals whose breathing has been compromised by cervical SCI.
Theophylline, which relaxes the smooth muscles of the bronchi, has been
used for decades to treat asthma and bronchitis. As a result, considerable
clinical data and history supports its safe use, which means we don’t have
to start from the beginning of the arduous, clinical trials, regulatory
process when considering theophylline’s SCI potential.
A key force behind developing theophylline’s
potential to enhance respiratory function after SCI has been Dr. Harry
Goshgarian and colleagues (Detroit, Michigan). His team has carried out
much of the supporting animal research, further delineated the crossed
phrenic pathway by which theophylline mediates its effects after SCI, and
initiated human studies.
Biochemistry: To better understand
theophylline’s mechanism of action, it is helpful to review some
biochemistry. Found in coffee and tea, theophylline is closely related to
caffeine, a molecule in which three methyl groups are bound to the
nitrogen atoms of a xanthine ring. When caffeine is metabolized and loses
a specific methyl group, theophylline is formed.
Theophylline blocks respiration-inhibiting, adenosine
receptors (i.e., it is an antagonist). Like a key that can fit in but
can’t turn a lock, theophylline is similar enough in structure to adenine
to block access to the receptor but different enough so as not to activate
the receptor. Theophylline specifically blocks phosphodiesterase, an
enzyme that degrades cAMP, resulting in an increase of intracellular cAMP.
Animal studies indicate that elevated cAMP levels 1) help growing axons to
overcome CNS-growth inhibitors and 2) increase the excitability of
presynaptic cation channels.
Crossed Phrenic Pathway: Theophylline
activates the crossed phrenic pathway, a normally dormant, backup pathway
that apparently restores some respiratory function after cervical SCI
(reviewed by Goshgarian in J Appl Physiol 94(2), 2003). Because
this pathway is located in a different part of the spinal cord as the
primary respiratory pathway, an injury that damages the primary pathway
may spare the backup pathway. Routinely, this backup pathway is not
active; i.e., although there is an intact neuronal connection between the
brain and diaphragm, no impulses are transmitted through it.
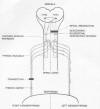
The key aspects of the crossed phrenic pathway are
illustrated in the following diagram (posted on
www.med.wayne.edu/anatomy/goshgarian).
In this diagram (click on diagram), the injury is a
spinal hemisection, an injury used in much of Goshgarian’s supporting
animal research. Such a hemisection located on the spinal cord’s left side
will paralyze the left hemidiaphragm.
The nerve impulses that contract the diaphragm
originate in brain nerve cells (called rVRG for rostral division of the
ventral respiratory group). These neurons connect to phrenic nucleus
neurons located on each side of the C3-5 spinal cord region. In turn,
these phrenic nucleus neurons transmit the contraction impulses to the
diaphragm.
Some of the neurons emanating from the brain split
off and cross over to the spinal cord’s other side before connecting to
the phrenic nucleus neurons. Hence, even though an injury may interrupt
the main nerve pathway, these cross-over nerves can be spared, maintaining
a direct, albeit inactive, connection from the brain to the paralyzed
hemidiaphragm.
Theophylline sufficiently stimulates the usually
dormant crossed phrenic pathway to activate the phrenic nucleus neurons,
allowing function-restoring impulses to reach the paralyzed hemidiaphragm.
In animal studies, Goshgarian and colleagues have
shown that theophylline-stimulated recovery lasted as long as 30 days
after the animals were weaned from the drug and drug serum levels were
undetectable. Due to these and other observations, Goshgarian concluded
that the persistent improvements in respiration after weaning from the
drug was due to some sort of theophylline-induced, neuronal plasticity. In
other words, theophylline’s respiration-enhancing benefits apparently are
not merely due to blocking adenosine receptors.
Human Studies: Goshgarian’s team has
studied theophylline’s effects on a number of individuals with cervical
injuries, including the following:
1) An individual with an asymmetric C5-7, 20-year-old
injury was given theophylline both intravenously in an acute study and
orally with pills for several weeks at home (Neurorehabil Neural Repair
13, 1999; also www.med.wayne.edu/anatomy/goshgarian).
Goshgarian reports: “In both the acute and chronic
study, there was significant improvement of respiratory muscle function in
this patient by as much as 172 percent. The patient's total inspiratory
muscle force … increased by 25 percent in the chronic study.”
“Furthermore, in another test indirectly measuring
the extent of breathing impulses that flow from the brain to the spinal
cord (descending respiratory drive), the patient improved in both the
acute and chronic study during both quiet and maximal breathing by 20-171
percent after the drug was administered.”
2) In a recent case, (Chest, 127(2), 2005), a
male who had sustained a cervical injury 30 years earlier from a gunshot
wound was weaned from his ventilator after theophylline treatment. The
patient was injured on the right side of his cord at the C5 level and on
the left side at the C6 level. He had been admitted to the hospital for
respiratory failure due to bacterial pneumonia and septicemia. Although
his infections were successfully treated, he continued to need ventilator
support until treated with theophylline about a month later.
Over the course of one day, theophylline was
intravenously administered. After administration, various parameters of
respiratory function showed improvement, and the patient was weaned from
ventilatory support the same day that the drug was given.
3) In a recent double-blinded,
placebo-controlled, crossover trial, Goshgarian and colleagues treated 10
patients with cervical SCI with theophylline (J Spinal Cord Med,
29(3), 2006). Although the overall results
for the entire group indicated no significant difference between placebo
and theophylline, 40 % of subjects showed marked improvement in
respiratory parameters (private communication). The extent of injury
appears to be more important than the level of injury in predicting theophylline’s beneficial effects. As discussed previously, for
theophylline to exert a beneficial influence, latent respiratory pathways
must be spared from injury.

4) Lithium:
Drs. Yat-wa Wong and Wise Young and colleagues have created a
network of Chinese SCI centers to carry out clinical trials on promising
therapies. Initia l network studies focus on the use of lithium and
umbilical-cord-blood-derived stem cells. These two approaches are being
considered together because evidence suggests that lithium stimulates
these cells to proliferate and to generate beneficial neuronal growth
factors.
l network studies focus on the use of lithium and
umbilical-cord-blood-derived stem cells. These two approaches are being
considered together because evidence suggests that lithium stimulates
these cells to proliferate and to generate beneficial neuronal growth
factors.
A low molecular-weight element used to treat
bi-polar disorder or manic depression, lithium exerts many
nervous-system effects through a number of potential physiological
mechanisms. Because lithium’s therapeutically beneficial doses are only
slightly lower than the levels considered toxic, a preliminary phase-1
study was carried out evaluating lithium’s safety and pharmacokinetics
(e.g., its absorption, distribution, elimination, etc) in 20 subjects
with either complete or incomplete chronic injuries. Subjects received
the lithium in approximately the same oral dose used to treat manic
depression. Ninety-five adverse events, mostly involving
gastrointestinal upset or nausea, were documented over the six-week
study period, and, as a result, seven subjects dropped out of the study.
Young has concluded that “the drug was clearly safe and did not produce
any significant adverse effects.”
The investigative
team has completed a larger phase-2 trial, which randomized 40 subjects
with chronic SCI to a six-week regimen of lithium or a placebo. Unlike
the earlier trial, only two subjects dropped out of this study. At the
end of the study, no difference was found in neurological outcomes
between lithium- and placebo-treated individuals. Unexpectedly, the
lithium-treated subjects had less neuropathic pain, a common problem for
those with SCI. This reduction persisted for six months after the end of
the study.
As a next-step continuation of these studies, a
phase-2 trial has been initiated assessing the effects of implanting
increasing amounts of umbilical-cord-blood-derived stem cells into the
cord above and below the injury site in individuals with chronic SCI
combined with lithium treatment. Finally, in a phase-3 trial, the
investigators intend to implant the stem cells into 400 subjects
randomized to receive either placebo or lithium for six weeks.
Similar studies are planned in the
United States and India.
The lithium focus is based on a number of promising
animal studies. First, Dr. L.W. Yick and colleagues (Hong Kong)
treated rats with experimental SCI with both lithium and an enzyme
called chondroitinase that digests a key molecular component of the
regeneration-inhibiting, injury-site scar. The treated rats demonstrated
more axonal regeneration and forelimb movement. Second, Dr. H. Su
et al (Hong Kong) showed that clinically relevant levels of lithium
enhanced the proliferation of neural progenitor cells grafted into the
rat spinal cord and reduced the post-injury, regeneration-inhibiting,
immune response. Finally, Dr. F. Fornai and associates (Italy)
demonstrated that lithium delayed the progression of the
neurodegenerative disease amyotrophic lateral sclerosis (ALS) in humans,
as well as mouse models of the disease.

5) Fibroblast
Growth Factor (FGF): Dr. Henrich Cheng and colleagues
(Taiwan) have treated nine patients with chronic, cervical injuries with
fibroblast growth factor.
 Numerous animal studies suggest that FGF
enhances regeneration after injury by promoting injury-site
revascularization, survival of injured neurons, and axonal growth. Of
the nine, eight were men, age ranged from 25 to 63 years (mean 48), and
the time lapsing since injury varied from five months to 8.5 years (mean
2.6). Six patients had been injured by motor vehicle accidents, two by
falls, and one by gunshot.
Numerous animal studies suggest that FGF
enhances regeneration after injury by promoting injury-site
revascularization, survival of injured neurons, and axonal growth. Of
the nine, eight were men, age ranged from 25 to 63 years (mean 48), and
the time lapsing since injury varied from five months to 8.5 years (mean
2.6). Six patients had been injured by motor vehicle accidents, two by
falls, and one by gunshot.
Each patient’s spinal cord was exposed through a
cervical laminectomy and the cutting of the cord’s outer membrane. As
needed, the cord was decompressed and freed from various scar-tissue
impingements. FGF within a fibrin-glue matrix was applied to the cord,
allowing for the slow diffusion of this neuronal nurturing growth factor
into the injury site.
Functional status was assessed before surgery and
monthly afterwards for six months. Assessments evaluated activities of
daily living, functional ability, spasticity, motor power, sensation,
and pain. On a scale of 1-100, motor scores improve from 52 to 69,
pin-prick-sensation from 61 to 72, and light-touch-sensation from 57 to
72. In some patients, bowel and bladder function got better and there
was less pain. No changes were noted in daily-living activities or
spasticity. One patient improved on the ASIA impairment scale from grade
A (complete injury) to C (incomplete), and another from grade C to D
(i.e., increasing function). Eight patients improved in neurological
level; for example, one functioning at the cervical C5 level improved to
the thoracic T1 level. The investigators concluded: “Modest nerve
regeneration occurred in all 9 patients after this procedure without any
adverse effects.”
Although noting the inherent limitations of this
preliminary phase I study, the investigators believed that the results
justify more in-depth studies. Because of the intervention’s highly
invasive nature, no comparison controls were recruited. Given the
study’s limitations, it is difficult to ascribe the modest improvements
to FGF treatment, the surgical decompression or tissue-removing
procedures, or the aggressive-rehabilitation efforts initiated after
surgery.
In 2011, the investigators reported the results of
a much larger, more statistically powerful study, which recruited 60
individuals, 30 of whom had cervical injuries and 30 of whom had thoraco-lumbar
injuries. The ratio of men to women was three to one, and the
average time since injury was 26 years. Twenty-six patients had been
injured by motor vehicle accidents, 17 from falls, four from sporting
accidents, and 13 from other causes. The FGF-fibrin glue combination was
administered after laminectomy, as well as as a booster of the mixture
three and six months later via lumbar puncture.
Forty-nine patients completed the two-year trial.
In the cervical group, motor scores improved on average from 28 to 37,
and in the thoraco-lumbar group from 57 to 61, statistically significant
results. Light touch and pinprick sensory scores also demonstrated
modest but statistically significant improvements. Functional
improvements were further documented by the ASIA-impairment scale,
neurological level assessments, and functional independence evaluations.
Finally, the percentage of patients with cervical and thoraco-lumbar
injuries who became ambulatory increased from 3.4% to 13.8% and 18% to
36%, respectively. No adverse side effects were reported.
TOP