1) Dr. Fernando Ramirez
(Mexico)
2) Diacrin Corporation
(USA)
3) Dr. Hui Zhu
(China)
4) Drs. Masoumeh Firouzi,
Hooshang Sabveri (Iran)
5) Dr. Xian-Hu Zhou
(China)
6) Miami Project to
Cure Paralysis (USA)
1) Dr.
Fernando Ramirezíteam (Mexico),
starting in the early 1990ís, has transplanted blue-shark, embryonic
neuronal cells (i.e., xenogeneic transplantation) into the injured spinal
cord of 89 patients with SCI.
 His
approach evolved from live-cell therapies developed by European scientists
starting in the 1930s long before stem cells emerged as a hot scientific
topic. Although his work is still considered controversial, it is less so
today than when he started his work because of all the other
cell-transplantation work that has since been initiated. More recently,
the program has shifted to the use of umbilical cord stem cells (www.ramirezdelrio.com).
His
approach evolved from live-cell therapies developed by European scientists
starting in the 1930s long before stem cells emerged as a hot scientific
topic. Although his work is still considered controversial, it is less so
today than when he started his work because of all the other
cell-transplantation work that has since been initiated. More recently,
the program has shifted to the use of umbilical cord stem cells (www.ramirezdelrio.com).

2) The
Diacrin Corporation (USA)
sponsored another xenotransplantation clinical trial. Specifically, Drs
John McDonald (St. Louis, Missouri) and Darryl DiRisio (Albany,
New York) injected about 14-million, immature, fetal pig, myelin
cells into the cord surrounding the injury site of 10 patients with
chronic SCI. The purpose of these cells was to remyelinate neurons that
have lost their insulating myelin sheaths due to injury, in turn,
restoring conduction potential. Because the expression of immune-provoking
proteins located on the porcine cell surface was altered, the need for
immune-suppressing drugs was supposedly greatly reduced.
Few results were reported for this clinical trial.
According to a December 2002 investment report, the survival of the
transplanted porcine cells was minimal, even with immunosuppressant drugs.

3) Dr. Hui Zhu
(China): As reported at the 1st International SCI Treatments &
Trials Symposium, Hong Kong, December 2005, Dr. Hui Zhu and
colleagues have transplanted fetal Schwann cells into 40 individuals with complete paraplegia. Thirty-two were men and eight were
women; age ranged from 18-58 (average 31) years; and time between injury
and transplantation ranged from 1-19 years, i.e., all were chronic
injuries. Patients were followed for 3-24 months using ASIA assessments, MRI imaging, and various electrophysiological evaluations.
Procedurally, Schwann cells cultured from fetal human
sciatic nerve (a nerve that runs through upper leg) were implanted into
the patientís injury cyst or cavity. After surgery, patients received
ambulatory training. Although a few regained substantial function, in
most patients improvements were slight as measured by ASIA evaluations of
motor function and light and deep sensation. In some patients, the MRI and
electrophysiological assessments indicated partial recovery of spinal-cord
function.
At the aforementioned symposium, Zhu
showed a case-study video of a young man who regained substantial function
due to the intervention.

4) Drs.
Masoumeh Firouzi, Hooshang Sabveri and colleagues (Iran) have also transplanted Schwann cells into the injury site. As
indicated earlier, these neuronal support cells remyelinate axons in the
peripheral nervous system, which, unlike the central nervous system has
considerable inherent regenerative potential.
This human trial builds upon on a recently published
study using rats with an experimental contused injury, the sort of injury
that is most common in humans. In
this study, Schwann cells were injected into the subarachnoid space
surrounding the spinal cord. Compared to control animals, the treated
rats regained more locomotion after injury and had more spared axons.
Given these results, the investigators received
permission from the Tehran University of Medical Sciencesí ethical
committee to transplant Schwann cells into 20 patients with SCI. The cells
were isolated from a sural nerve in back of the patientís calf (i.e., autologous
cells with no rejection potential) and cultured in a sterile laboratory
for two to five weeks. The cultured cells were then implanted back into
the injury site.
Initially, nine of the 20 authorized patients were
treated. This study excluded individuals older than 50, whose cord has
been transected, and whose injury site is considered too extensive. A
number of the treated patients had long-term chronic injuries, including
patients 16, 23, 25 years post injury. Using the data coming out this
preliminary study, more definitive criteria will be developed on the most
appropriate post-injury, treatment window.
Three to six months after implantation, no adverse
side effects have been observed. According to Dr. Firouzi, all treated
patients ďhave shown some degree of sensory recovery; most have motor
recovery findings using quantitative (digital) motor evaluations; and some
showed sphincter and sexual improvement, all compared to the patientís
steady-state before the transplantation. Three of them can walk now (two
using parallel bars and one using walker).Ē The investigators note that
progress should continue for some time after this limited follow-up
period.
At that point in time, preliminary preparations were
being made to develop facilities and train other doctors to make the
procedures more accessible to the general SCI community.
In 2008, the investigators published the results of
treating four patients with mid-thoracic injuries with autologous
Schwann cells. Age ranged from 22 to 43 y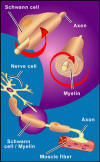 ears, and they had been injured
28 to 80 months before treatment. As indicated before, the patientís sural nerve was the source of the transplanted Schwann cells. The
patients were followed for one year using ASIA-impairment, sphincter,
sexual-function, and MRI assessments. Of the four patients, only one
with an incomplete injury, who had extensively rehabilitated,
demonstrated motor and sensory recovery one year post transplantation.
No adverse effects were observed. From these preliminary findings, the
investigators concluded that ďautologous Schwann cell transplantation is
generally safe for the selected number of SCI patients but it does not
prove beneficial effects.Ē
ears, and they had been injured
28 to 80 months before treatment. As indicated before, the patientís sural nerve was the source of the transplanted Schwann cells. The
patients were followed for one year using ASIA-impairment, sphincter,
sexual-function, and MRI assessments. Of the four patients, only one
with an incomplete injury, who had extensively rehabilitated,
demonstrated motor and sensory recovery one year post transplantation.
No adverse effects were observed. From these preliminary findings, the
investigators concluded that ďautologous Schwann cell transplantation is
generally safe for the selected number of SCI patients but it does not
prove beneficial effects.Ē
In 2011, the
investigators reported two-year follow-up results of 33 individuals
treated with transplanted autologous Schwann cells as described above.
With an average age of ~ 33 (range 23-50) years and duration of injury
4.1 years, 22 were men and 11 women. Twenty-four and nine patients had
thoracic and cervical injuries, respectively. All had either
ASIA-A-complete or ASIA-B (some sensory function) injuries (see
appendix). Follow-up results indicated that the transplantation
procedures were generally safe. Small improvements were observed in 1)
light-touch sensation, especially in those with cervical lesions, and 2)
motor function, particularly in those with more recent cervical
injuries. Several patients reported new urinary and/or bowel sensation
and control.
5) In 2012,
Dr. Xian-Hu Zhou and colleagues (China) reported treating six
individuals with SCI with autologous (i.e., from the patient) Schwann
cells. All except one were men, age ranged from 7 to 34, and the time
between injury and transplantation varied from one week to 20 months. A
Schwann-cell-containing sural nerve segment was surgically removed from
the patientís leg, the resulting tissue incubated in culture, and the
Schwan cells isolated. After exposing the cord, the Schwann cells were
injected into the area adjacent to the injury site. The patients were
followed for at least five years using a variety of assessments.
Although the study had limitations due, in part, to its small size, all
patients showed some signs of improvement in autonomic, motor, and
sensory function.
6)
Investigators at the
Miami Project to Cure Paralysis
(USA) have received permission
from the Food and Drug Administration to initiate a phase 1 clinical
trial assessing the safety of transplanting autologous Schwann cells
into eight patients with acute thoracic injury. To obtain the Schwann
cells, a biopsy of sensory nerve in the leg will be carried out, and the
resulting tissue cultured for three to five weeks to obtain the
necessary number of cells for transplantation. All subjects will be
followed for one year.
TOP