1) Omental Transposition
2) Omental
Transplantation
1)
Omental Transposition: Dr. Harry Goldsmith (Nevada, USA)
pioneered the development of omental transposition procedures for various
central nervous system disorders.
 His
work has stimulated many others who have now treated thousands of patients
for SCI and other neurological disorders, such as stroke, cerebral palsy,
Alzheimer’s disease, and Parkinson’s disease. The procedure’s acceptance
has grown in other parts of the world, such as in China where many
individuals have had function-restoring omental surgery.
His
work has stimulated many others who have now treated thousands of patients
for SCI and other neurological disorders, such as stroke, cerebral palsy,
Alzheimer’s disease, and Parkinson’s disease. The procedure’s acceptance
has grown in other parts of the world, such as in China where many
individuals have had function-restoring omental surgery.
Physiology: The omentum is a highly vascular,
fatty tissue approximately 14-inches long and 10-inches wide that hangs
like an apron over the intestines and lower abdomen area. Although the
omentum has been viewed as an inert tissue bereft of significant
biological function, scientists are now discovering that it is an
intriguing, physiologically dynamic tissue with a considerable body of
research that supports its therapeutic potential (see specifically, Agner
et al, Neurological Research, January, 2001 and The Omentum
Application to Brain and Spinal Cord, edited H.S. Goldsmith, Forefront
Publishing, 2000):
·
Blood supply: The omentum contains angiogenic factors
that stimulate the growth of new blood vessels into whatever tissue it is
surgically placed next to, including the brain and spinal cord.
·
Lymphatic System: The omentum is rich in lymphatic
vessels and tissue that are critical in removing metabolic waste and
excess fluid, destroying toxic substances, and fighting disease.
·
Immune System: Omental areas called “milky spots” are
capable of generating specialized immune cells that facilitate healing.
For example, some believe that the migration of omental immune cells can
help repair injured spinal cords.
·
Edema Absorption: The omentum’s lymphatic system has
an enormous capacity to absorb edema fluid, including that associated with
spinal cord swelling.
·
Source of Biological Material: The omentum is a rich
source of biological material that enhance tissue growth, including
angiogenic factors, key neurotransmitters, nerve growth factors, and
agents involved in inflammatory and immune processes.
·
Stem Cells: Evidence suggests that omental tissue
contains stem cells - omnipotent master cells that can differentiate into
a variety of cell types. For example, Dr. Ignacio Garcia Gomez (Madrid,
Spain) and colleagues demonstrated the presence of stem cells in the human
omentum (Neurological Research, 27, December 2005). These cells
were shown to synthesize key growth factors that promote vascularization
when transplanted.
Transposition Surgery: In a six-hour
operation, surgeons cut in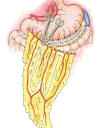 to
the abdominal cavity to access the omentum, which is then gently separated
from the colon and the stomach in a way that maintains blood and lymphatic
circulation. It is then surgically tailored to create a pedicle – a piece
of connected tissue of sufficient length with intact circulation to reach
the injury site, like a square handkerchief would be cut to make a long
necktie. The omental ped
to
the abdominal cavity to access the omentum, which is then gently separated
from the colon and the stomach in a way that maintains blood and lymphatic
circulation. It is then surgically tailored to create a pedicle – a piece
of connected tissue of sufficient length with intact circulation to reach
the injury site, like a square handkerchief would be cut to make a long
necktie. The omental ped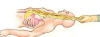 icle
is then tunneled underneath the skin, placed over the exposed cord, and
sutured to the cut edges of the dural membrane surrounding the cord.
icle
is then tunneled underneath the skin, placed over the exposed cord, and
sutured to the cut edges of the dural membrane surrounding the cord.
Because creating the omental pedicle can be tricky,
some surgeons use a substitute procedure, in which a free, unattached
piece of omental tissue is surgically placed over the injured cord and
connected to a surrounding vascular source. Although blood circulation is
maintained, because the graft is separated from the omentum’s lymphatic
system, the tissue’s ability to absorb fluid is eliminated.
Goldsmith estimates that about 40% of omental SCI
patients have regained some function; Chinese surgeons have reported an
even greater improvement rate.
Criticism: A 1996 study (Clifton, et al,
Spinal Cord, 34, 1996) appeared to provide the evidence to dismiss
omental transposition as a viable SCI treatment. In this study, 11
patients with SCI were examined a year after omental surgery. Results were
inconclusive; some subjects improved, and others did not. Because these
ambiguous results were associated with side effects, the investigators
concluded that there was “no justification for further clinical trials of
this procedure.”
However, soon after Goldsmith rebutted this criticism
(Spinal Cord, 35, 1997). Specifically, Goldsmith noted that the
investigators had used two different surgical procedures, automatically
confounding the study. Over half the time, they had used a free omental
tissue graft instead of, as stated in their objectives, an attached
omental pedicle. By so doing, they eliminated the tissue’s beneficial
fluid-absorbing capability.
Furthermore, although the study’s goal was to
determine the specific effect of the omentum placed directly on the
injured cord, the final analysis included outcomes of several patients
whose omental graft was shown not even to be physically attached to the
cord or had been surgically removed before analysis. In other words, they
had factored in results that were not applicable to the stated study
objectives, and, hence, significantly skewed the reported results.
Collagen/Omentum Case Study: In 2005,
Goldsmith and colleagues reported the use of an omental/collagen bridge to
help restore function in Andrea a young German woman who became paraplegic
3½ years earlier from a skiing accident (Neurological Research 27,
2005). Andrea’s post-injury MRI indicated a near total spinal cord
transection at the thoracic T6-7 level. Three and half years after injury,
Andrea underwent surgery in which the scar tissue that now filled the 4-cm
gap in her cord was replaced with an omental-collagen bridge. After
removal of the scar tissue, 4-5 cc of collagen, a reverse polymer that
hardens at body temperature, was delivered into this gap, and after
hardening, an omental pedical was sutured over the collagen bridge.
Several years after the surgery, Andrea started an
aggressive physical rehabilitation at the
Neuro Institute (Phoenix, AZ) managed by Arnie Fonseca, a co-author.
Since her surgery, she has regained considerable function below the injury
level, including some ambulatory ability.
The article includes a series of MRIs taken
immediately after injury and after construction of the omental-collagen
bridge; and 1, 2, 3, 4, 5, and 6 years after surgery. These
time-sequential MRIs demonstrate ongoing development of axonal structure
connecting the proximal and distal spinal cord segments.
Proposed Procedures for Acute SCI: In 2007,
Goldsmith proposed that the acute injury phase may be the most optimal
time to place the omental pedical over the injury site (Neurological
Research, 29, 2007). As noted above, the omentum’s huge capacity to
absorb fluid could potentially reduce neurological damage associated
with spinal-cord swelling at the time of acute injury. The fluid that
accumulates at the injured cord promotes scar development, resulting in
the constriction of nearby capillaries and, in turn, healing-inhibiting
ischemia (i.e., compromised blood flow). Specifically, the omentum’s
absorption of edema fluid would lower the levels of fibrinogen, the
protein from which blood-clot-forming fibrin is generated, resulting in
less scar-tissue formation. Although the spinal cord is often
surgically decompressed soon after injury to remove impinging tissue or
bone fragments, this procedure does not necessarily release the
swelling-related pressure underneath the spinal-cord membrane. At the
time of surgical decompression, Goldsmith suggests that a
pressure-releasing incision be made in this membrane followed by the
placement of a fluid-absorbing omental pedical over the now-exposed
cord.
Dr. Himanshu Bansal (India) and
colleagues have incorporated Goldsmith’s omental transposition ideas
into their growing therapeutical armamentarium for acute SCI.
Specifically, they have placed a fluid-absorbing omental pedical over
the exposed cord of several individuals with thoracic injuries. Because
the cord was lacerated in these injuries, decompression was not required
as is often the case with contusion injuries.
Although recognizing it is difficult to assess
treatment-related improvements in the acute-injury phase, Bansal will
attempt to get insights on effectiveness by  comparing
treated patients with untreated individuals with comparable injuries. He
will also carry out various follow-up tests six months afterwards,
including neurological assessments, evaluation of injury-site MRI’s, and
electrophysiological measurements of nerve conduction.
comparing
treated patients with untreated individuals with comparable injuries. He
will also carry out various follow-up tests six months afterwards,
including neurological assessments, evaluation of injury-site MRI’s, and
electrophysiological measurements of nerve conduction.
Bansal intends to perform these omental
transposition procedures on more patients in the future. The timing of
his procedures will depend upon the nature of the injury as determined
by MRI assessments: 1) pure contusion, 2) less than 50% of the cord
lacerated, 3) more than 50% but not all of the cord lacerated, and 4)
complete transection of the cord.
For example, in the case of pure contusion
injuries, he intends to carry out decompression and transpose the
omentum immediately after injury; with lacerative injuries, he will wait
several weeks.
2)
Omental Transplantation: Many others have used omental
transplantation, not transposition, including
Dr. Carl Kao and Dr. Hernando Rafael
(Mexico).
 As
reported at the 2001 WHO-sponsored conference held in Reykjavik, Iceland, Rafael grafts an unattached piece of omental
tissue over the injured cord and connects it to a surrounding vascular
source. At the time of the conference, he had treated 232 patients with
traumatic SCI with the procedure. He claimed that 43 percent have
neurologically improved, including 43 who are walking with or without the
use of orthopedic devices. Somewhat similar omental transplantation
procedures were reported by Moscow’s Dr. Georgie Stepanov.
As
reported at the 2001 WHO-sponsored conference held in Reykjavik, Iceland, Rafael grafts an unattached piece of omental
tissue over the injured cord and connects it to a surrounding vascular
source. At the time of the conference, he had treated 232 patients with
traumatic SCI with the procedure. He claimed that 43 percent have
neurologically improved, including 43 who are walking with or without the
use of orthopedic devices. Somewhat similar omental transplantation
procedures were reported by Moscow’s Dr. Georgie Stepanov.
TOP