1) Dr. Giorgio Brunelli
(Italy)
2) Dr. Shaocheng Zhang
(China)
3) Dr. A Livshits
(Russia & Israel)
4) Drs. Chuan-Guo Xiao & Kenneth
Peters (China & USA)
5) Dr. Haodong Lin
(China)
6) Dr. Justin Brown
(USA)
7) Dr. Marc Tadie
(France)
8) Dr. Carl-Axel Carlsson
(Sweden)
9) Dr. Hiroyasu Makino
(Japan)
10) Dr. L. W. Freeman
(USA)
11) Dr. A.
Chiasserini (France)
12) Drs. Charles Frazier &
Charles Mills (USA)
123 Dr. Basil Kilvington
(Australia)

Peripheral-nerve rerouting represents an surgical
procedure that has considerable documented potential for restoring
significant function after SCI. Often with this procedure, peripheral
nerves (i.e., those outside of the spinal cord and brain) emanating from
the cord above the injury site are surgically rerouted and connected to
those below the injury site. This reestablishes a functional neuronal
connection from the brain to previously dormant muscle or sensory
systems.
In spite of intimidating neuroanatomical
terminology, peripheral-nerve rerouting is conceptually relatively easy
to understand. For example, visualize a house in which the power to the
back bedroom is lost (i.e., area below the injury) due to a burned-out
master electrical cable (i.e. the spinal cord injury). Instead of fixing
the master cable, you disconnect the wire that powers the living-room
television (i.e., a nerve to the rib or wrist region), perhaps attach an
extension cord, tunnel it through the walls, and splice it directly to
the bedroom wiring, circumventing the damaged section of master cable.
Amazingly, as discussed below, these
function-restoring surgeries have been around in some form for nearly a
century - but relegated to the therapeutic dustbin until recently.

1)
Dr. Giorgio Brunelli (Italy)
has surgically rerouted peripheral nerves to bypass the injury site,
reestablishing a functional neuronal connection from the brain to
previously dormant body areas. For example, he has restored some function
by redirecting the wrist’s ulnar nerve and connecting it to nerves that
control leg functioning below the injury site. After this procedure, a patient with a
complete spinal-cord transection could stand up and walk short distances.
In another procedure carried out in a woman with a complete thoracic
transection, the peroneal nerve (a nerve to the leg) was used as a bridge
directly from the spinal cord above the injury site to the nerves of the
gluteus and quadriceps muscles. After two years, she was able to walk
30-40 meters with a walker. (Photo: Dr.
Giorgio Brunelli & Nobel Laureate Dr. Rita Levi-Montalcini)
functioning below the injury site. After this procedure, a patient with a
complete spinal-cord transection could stand up and walk short distances.
In another procedure carried out in a woman with a complete thoracic
transection, the peroneal nerve (a nerve to the leg) was used as a bridge
directly from the spinal cord above the injury site to the nerves of the
gluteus and quadriceps muscles. After two years, she was able to walk
30-40 meters with a walker. (Photo: Dr.
Giorgio Brunelli & Nobel Laureate Dr. Rita Levi-Montalcini)
Because the second procedure represents a direct
peripheral-nerve to spinal-cord connection, it challenged traditional
beliefs on how neurons control muscle function. Specifically, upper motor
neurons (nerves within the spinal cord) and lower motor neurons (nerves
that leave the cord to connect to muscles) use different
neurotransmitters. Hence, theoretically, the muscle should not be
triggered due to neurotransmitter incompatibility. However, Brunelli has
recently shown that target muscles are genetically reprogrammed, producing
receptors that are responsive to the neurotransmitters released by the
upper motor neurons that have grown to the muscles through the peripheral
nerve bridge.

2)
Dr. Shaocheng Zhang (China),
through a variety of procedural permutations, has rerouted peripheral
nerves to restore function in hundreds of patients with SCI. Restored
function depends upon the specific functions that the target nerves serve
(e.g., leg muscle function, bladder and bowel control, sensation, etc).
For example, the rerouted nerve could be connected to a nerve that
controls urination, or it could be reconnected to nerve that controls
upper leg muscles. Many possible rerouting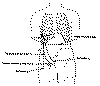 arrangements exist. Zhang commonly reroutes one of the intercostal nerves
that lead from the spinal cord around each rib to the sternum. If the
intercostal nerve is not long enough to reach the target nerve site below
the injury level, a sural nerve segment is attached to the intercostal
nerve (Click on thumbnail).
arrangements exist. Zhang commonly reroutes one of the intercostal nerves
that lead from the spinal cord around each rib to the sternum. If the
intercostal nerve is not long enough to reach the target nerve site below
the injury level, a sural nerve segment is attached to the intercostal
nerve (Click on thumbnail).
If the injury site is above the thoracic area where
the intercostal nerves originate, other peripheral nerves can be selected.
For example, in several cases, Zhang has rerouted the ulnar nerve.
In addition to the intercostal and ulnar nerves,
peripheral-nerve-rerouting options can restore function for virtually any
level of injury. For example, in high-level injuries, functional
peripheral nerves above the injury site (e.g., cervical plexus nerve
branches originating from the higher cervical regions) can be connected to
nearby dysfunctional nerves below the injury site (e.g., brachial plexus
nerves originating from the lower cervical regions), potentially restoring
respiratory ability to a previously ventilator-dependent quadriplegic.
Zhang et al have reported the results of 23 patients
who had an intercostal nerve surgically rerouted to nerve roots below the
injury site.
Specifically, two to four intercostal nerves were transferred to the
vertebral canal through a submuscle tunnel and connected to lumbar nerve
roots. If the selected intercostal nerve was of insufficient length to
reach the specific lumbar region, a sural nerve segment was attached. The
23 patients included 19 males and 4 females, ranged in age from 19 to 45,
were traumatically injured between the thoracic T9-12 levels, and had
sustained their injuries 6 to 30 months before surgery. Of the 23
patients, 18 regained some ambulatory function and were able to walk with
crutches or other assistive technology.
Zhang and colleagues have also used an
intercostal-sural nerve bridge to restore some bladder and bowel function.
Specifically, two intercoastal nerves above the injury site were
transferred to the vertebral canal through a submuscular tunnel. A sural
nerve segment was sutured to the intercostal nerves and then to the S2-4
nerve roots. Of the 30 patients studied, 19 were male and 11 female, ages
ranged from 19 to 46, and 17 and 13 traumatically injured in the T9-11 and
T12-L2 level, respectively. Significant bowel and bladder function was
restored in the majority of patients.
Zhang’s various nerve-rerouting surgeries are listed
below. Although the neuroanatomical terminology can be intimidating, the
fundamental concept is theoretically simple: a functioning nerve from
above the injury site is rerouted and connected to a paralysis-affected
nerve.
C1-4 Injuries:
A) The accessory nerve arising
from a cranial nerve is connected to the paralysis-affected phrenic nerve,
restoring some respiration.
B) The facial nerve’s
cervical branches are connected to the paralysis-affected phrenic nerve,
restoring some respiration.
C5-8 Injuries:
A) The accessory nerve is
connected to the paralysis-affected musculocutaneous nerve, restoring some
bicep function.
B) To restore some hand
function, the accessory nerve is connected the paralysis-affected median
nerve, and a still-functioning cervical plexus nerve branch is connected
to a paralysis-affected brachial plexus branch.
C) Again to restore some
hand function, a connection is made between a pectoral nerve below the
injury site to the paralysis-affected ulnar and radial nerves.
T2-7 Injuries:
A) The arm’s functional
ulnar nerve is rerouted below the injury site to paralysis-affected
femoral and ilioinguinal nerves, restoring some ambulation and pelvic-area
sensation.
B) The arm’s ulnar nerve is
attached to paralysis-affected femoral and obturator nerves, restoring
some leg-muscle function.
T8-11 Injuries:
A) Rib-associated
intercostal nerves are rerouted and connected to the paralysis-affected
femoral cutaneous and ilioinguinal nerves, restoring some leg and sexual
sensation.
B) Intercostal nerves are
rerouted and connected to paralysis-affected lumbar nerve roots, restoring
some walking ability.
C) Intercostal nerves are
rerouted and connected to paralysis-affected sacral nerve roots, restoring
some bowel-and-bladder function.
Cauda Equina Injuries:
A) Intercostal nerves are
rerouted and connected to the paralysis-affected pudendal nerve, restoring
some bowel-and-bladder function.
B) Still-functioning gluteal
nerves are connected to the nearby, paralysis-affected pudendal nerve,
restoring some bowel-and-bladder function.
C) The leg’s saphenous nerve
is connected to the tibial nerve, restoring some sensation to the foot.
D) The leg’s sural nerve is
connected to tibia nerve, restoring some sensation to the foot’s plantar
region and toes.

3)
Dr. A Livshits et al (Russia)
have connected intercostal nerve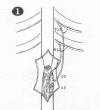 s
above the spinal cord injury site to nerve roots below the injury site in
11 patients with complete L1 injuries.
All patients were male, ranged in age from 18-47 years, and sustained
their injuries 1-4 years before surgery. Specifically, intercostal nerves
from the 11th and 12th rib were transferred through a vertebral
canal created under deep spinal muscles. These nerves were then connected
end-to-end to S2-3 nerve roots that had been cut in their proximal
portion. (Thumbnail illustration from Spinal Cord 42(4), 2004)
s
above the spinal cord injury site to nerve roots below the injury site in
11 patients with complete L1 injuries.
All patients were male, ranged in age from 18-47 years, and sustained
their injuries 1-4 years before surgery. Specifically, intercostal nerves
from the 11th and 12th rib were transferred through a vertebral
canal created under deep spinal muscles. These nerves were then connected
end-to-end to S2-3 nerve roots that had been cut in their proximal
portion. (Thumbnail illustration from Spinal Cord 42(4), 2004)
Various bladder-function assessments were carried out
10-12 months after surgery, including bladder capacity, urine volume,
residual urine, detrusor tone, voiding pressure, and force of detursor
contraction. Restoration of reflex voiding occurred in all patients.

4) Dr. Chuan-Guo
Xiao and colleagues (Wuhan, China & New York, USA) have
rerouted nerves below the injury site, restoring the
patient’s ability to control urination through skin stimulation.
 As illustrated, the
lumbar-level L5 ventral nerve root is usually connected to the
sacral-level S3 (or S2) ventral nerve root. (The ventral and dorsal roots
contain nerves that leave and enter the spinal cord, respectively). After
rerouting, by scratching, gently squeezing, or electro-stimulation of the
skin associated with the L5 dermatome, a voiding response is initiated.
Basically, these actions trigger a sensory signal that enters the cord via
the L5-dorsal root
As illustrated, the
lumbar-level L5 ventral nerve root is usually connected to the
sacral-level S3 (or S2) ventral nerve root. (The ventral and dorsal roots
contain nerves that leave and enter the spinal cord, respectively). After
rerouting, by scratching, gently squeezing, or electro-stimulation of the
skin associated with the L5 dermatome, a voiding response is initiated.
Basically, these actions trigger a sensory signal that enters the cord via
the L5-dorsal root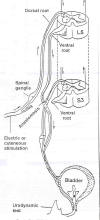 ,
in turn, stimulating nerves that leave the cord through the L5-ventral
roots now connected to the bladder-controlling S3-ventral nerve root.
Provided this area of rerouting is undamaged, the procedure is suitable
for all levels of injury. Because this procedure does not restore bladder
sensation, patients need to consciously initiate the triggering procedures
for urination.
,
in turn, stimulating nerves that leave the cord through the L5-ventral
roots now connected to the bladder-controlling S3-ventral nerve root.
Provided this area of rerouting is undamaged, the procedure is suitable
for all levels of injury. Because this procedure does not restore bladder
sensation, patients need to consciously initiate the triggering procedures
for urination.
In his 2003 article, Xiao reported the results of
treating 15 patients with complete, ASIA-A injuries with this procedure.
Injuries ranged from C4 to T12; in other words, all were well above the
nerve-rerouting area. The time between injury and surgery averaged 6.8
years, and average follow-up was three years. Of the 15 patients, 10
recovered bladder-storage and emptying function starting about a year
after surgery (the time for regenerating neurons to reach their target
site), residual urine decreased from 332 to 31 milliliters, and
urinary-tract infections became negligible. In addition, two patients
partially recovered, requiring electrical stimulation to initiate voiding,
and, although decreasing residual urine, still retaining over 100
milliliters. Of the three remaining patients, one was lost to follow-up,
and two did not accrue benefits, apparently due to poor rerouting
connections.
Before surgery, six of the 12 patients who eventually
recovered bladder control had elevated serum creatine levels, an
indicator of kidney problems. A year and half after the procedure, their
creatine levels returned to normal. In addition, patients who regained
bladder control also regained bowel control.
In a 2010 update
posted on a SCI-discussion forum, Xiao indicated that since 2000 he and
his associates have cumulatively treated 350+ patients with SCI and
1,500+ patients with spina bifida (birth defect which results in an
incompletely developed spinal cord). Overall success rate exceeded 80%.
In addition, to restoration of bladder and bowel function, he noted that
20-25% of the patients regained some sexual functioning. He believes
this sexual improvement is mainly due to the overall enhancement of the
patients’ physical condition after bladder and bowel function have been
normalized. To further disseminate his function-restoring procedures,
Xiao has started training neurosurgeons at a variety of locations in
North America and Europe.

Dr. Kenneth Peters and
associates (USA) have initiated a study evaluating Dr. Xiao’s
aforementioned procedures.
 Peters’
study recruited 12 subjects with either SCI or spina bifida, again, a birth
defect which results in an incompletely developed spinal cord. Because
lumbar-level nerves below the injury site are rerouted to even
lower level sacral nerves (see above), study subjects with SCI will be
required to 1) have injuries above the L1 level (i.e., thoracic or
cervical injuries) and 2) possess complete ASIA-A classified injuries
(see appendix). Bladder function, the primary outcome, will be evaluated
six months and one-year after nerve rerouting. Secondary outcomes
include bowel function, quality of life, activities of daily living, and
sexual functioning. Preliminary results
indicate that bladder and bowel function was improved in the majority of
the subjects.
Peters’
study recruited 12 subjects with either SCI or spina bifida, again, a birth
defect which results in an incompletely developed spinal cord. Because
lumbar-level nerves below the injury site are rerouted to even
lower level sacral nerves (see above), study subjects with SCI will be
required to 1) have injuries above the L1 level (i.e., thoracic or
cervical injuries) and 2) possess complete ASIA-A classified injuries
(see appendix). Bladder function, the primary outcome, will be evaluated
six months and one-year after nerve rerouting. Secondary outcomes
include bowel function, quality of life, activities of daily living, and
sexual functioning. Preliminary results
indicate that bladder and bowel function was improved in the majority of
the subjects.

5) In an effort to restore bladder function in
individuals with paraplegia, Dr.
Haodong Lin and associates (China) have rerouted ventral
nerve roots (i.e., nerves that leave the spinal cord) above the injury
site and connected them through an intervening nerve graft to
paralysis-affected sacral nerve roots that lead to the bladder. Of the
10 patients recruited, six were men and four were female; age ranged
from 22 to 53 (average 38) years; and the time lapsing since injury
varied from three to 14 (average 8.7) months. All patients had ASIA-A
motor and sensory complete injuries, three, five, and two with T12, L1,
and L2 injuries, respectively.
After transection, the T11 ventral nerve root was
connected to a 30-centimeter sural nerve segment (obtained from the
leg). The other end of this intervening segment was then attached to a
transected S2 ventral nerve root that connects to the bladder.
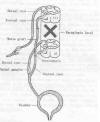
Seven of the 10 patients regained “satisfactory”
bladder control 18 to 24 months after surgery, corresponding to the time
it takes time for the transected T11 neuronal axons to grow to their
target site through the newly created pathway. Similar to Dr. Xiao’s
procedures discussed above, urination could be then triggered by
scratching or gently squeezing the skin area associated with the T11
dermatome for 5-10 seconds. This stimulation sends a sensory signal to
the cord through the T11-dorsal root (i.e., input), in turn, stimulating
nerves that leave the cord through the T11-ventral roots (output) now
rerouted to the bladder.
In the seven patients with positive outcomes,
residual urine volume in the bladder decreased from an average of 477
milliliters before surgery to 35 milliliters afterwards. In addition, as
patients recovered their ability to voluntarily empty their bladders,
the incidence of urinary tract infections greatly decreased. Finally,
five of the patients had elevated serum creatine levels before surgery,
an indicator of kidney problems, which returned to a normal range 24
months after surgery. Few adverse complications were observed.

6) In an effort to provide additional hand function
for individuals with cervical injuries,
Dr. Justin Brown
(USA) has developed surgical procedures for creating new connections
between still-functioning upper-arm nerves and paralysis-affected nerves
servicing the lower arm and hand. This intervention initially was
carried out in a 28-year-old male with a C5-level injury sustained 13
years earlier from a football accident. Due to his injury, arm function
was limited to the shoulders and biceps.
Four years after injury, he started using the
“Freehand” functional-electrical-stimulation (FES) device. As discussed
elsewhere, this device allows the user to artificially pinch and grip
through a system of embedded electrodes controlled by a
movement-sensitive device placed on the opposite shoulder. However,
because the patient felt that 1) the resulting hand control was limited,
2) the device was cumbersome, and 3) wires leading to electrodes caused
discomfort, he chose to have the system removed and consider other
opitions.
To reestablish voluntary control of various muscles
involved in hand function, several new nerve connections had to be
created. Although the procedures sound relatively straightforward in
principle, they require considerable surgical sophistication and ability
to identify nerves serving specific muscles.
The first new connection was created to restore
wrist and finger flexion. Specifically, segments (called fascicles) of
the musculocutaneous nerve, which controlled the still-functioning
biceps and related muscles, were connected to a segment of the median
nerve, leading to paralyzed forearm and hand muscles. Because only a
portion of the musculocutaneous nerve was redirected, function in the
bicep-related muscles already served by this nerve was not sacrificed.
Illustration A shows the presurgery situation
involving the musculocutaneous and median nerves and the muscles they
serve. Muscles under volitional control are colored red, while
paralysis-affected muscles are highlighted in gray. Illustration B shows
the location of the newly created musculocutaneous-median nerve
connection and, as can be seen by the expanded red coloring, the
additional key muscles that should come under volitional control as a
result of the connection.
At the time these nerve-rerouting procedures were
reported, functional outcomes in this patient were not available; i.e.,
it takes time for the nerves to regenerate to the target muscles. In
theory, however, the patient, who before the procedure only had
elbow-flexion and shoulder function, should recover his ability to
reach, grasp, and release. This functional recovery will be obtained
without sacrificing existing functions, which is often the case with the
more commonly used tendon-transfer procedures.

7) Dr. Marc
Tadie and colleagues (France) have
rerouted lumbar nerve roots from below the injury site to the
 spinal
cord above the injury site, creating a functional neuronal pathway from
brain to paralysis-affected leg muscles. This rerouting was undertaken in
man who sustained a clinically complete T9 injury at age 52 three years
earlier in an automobile accident.
spinal
cord above the injury site, creating a functional neuronal pathway from
brain to paralysis-affected leg muscles. This rerouting was undertaken in
man who sustained a clinically complete T9 injury at age 52 three years
earlier in an automobile accident.
Specifically, three 6-cm long, autologous nerve
segments were implanted on each side of the cord at the T7-8 level
immediately above the injury site. The segments were inserted 5 mm into
the cord to allow contact with ventral horn motor neurons but with care to
avoid injury of the main ascending and descending neuronal tracks. The
segments were sutured and glued to the spinal cord. The opposite ends were
sutured to L2-4 ventral nerve roots (i.e., containing the motor neurons
exiting the cord), which had been detached from the point that they exit
the cord. The graft-root connection was covered with a silastic tube.
Eight months after surgery, the patient was able to
initiate some contraction of adductor and quadriceps leg muscles. This
ability was confirmed by various electrophysiological assessments.

8) Drs.
Carl-Axel Carlsson and Torsten Sundin (Sweden)
rerouted thoracic nerve roots above the injury site to sacral nerves below
the injury site in two men with L1 injuries. The
patients, age 23 and 43, were acutely injured from accidents 10 and 14
days earlier. Specifically, T12-nerve roots were connected to the S2 and
S3 ventral and dorsal nerve roots that had been cut as close as possible
to the cord. The connection was made using a Silastic filter without
sutures. This rerouting theoretically created a functional connection
between the brain and bladder.
Approximately a year later, both patients, “could
feel the urge to void, could initiate micturition voluntarily, and could
empty their bladders satisfactorily.” In one patient, some psychogenic and
tactile erectile function was regained. Unlike previously discussed
rerouting procedures, the observed functional recovery occurred in a
post-injury phase when some recovery is not uncommon.
In a early case study, Carlsson and Sundin restored
bladder function in a four-year old girl with paraplegia due to a
lumbar-level myelomeningocele (i.e., a spinal bifida birth defect in
which the cord and membranes protrude from the back). The myelomeningocele was surgically excised in a fashion in which
no neuronal connection remained between the cord above the lesion and
nerve roots below the lesion. A pair of T10 or T11 ventral nerves roots
were cut close to where they exited the dura and connected end-to-end to
S1 and S2 ventral roots using a Millipore microfilter. Eight-months later,
the girl was able to voluntarily urinate.

9) Dr.
Hiroyasu Makino (Japan): Based on Dr.
Freeman’s operative techniques discussed below, Makino et al routed freed
intercostal nerves above the injury site to areas below the injury site in
eight patients with paraplegic injuries sustained at least a year before
surgery. In four patients, one
pair of T10 or T11 intercostal nerves was inserted in the conus medullaris
(i.e., the spinal cord’s conical tip) and another pair connected to L4
nerve roots. In four other patients, two pairs of intercostal nerves were
connected to L3 and L4 nerve roots.
At the time of this brief, the follow-up period to
assess patient functional improvement was relatively limited. As a result,
only one patient, who had sustained a L1-L2 injury 13 months before
surgery, demonstrated significant improvement, including the ability to
walk with the assistance of parallel bars.

10) Dr. L. W. Freeman
(Indiana, USA): Moving back further in time
to 1951, Freeman connected intercostal nerves above the injury site to
sacral nerve roots below the injury site. This surgical procedure was
carried out in a 33-year-old male injured near the T8-9 level from
police gunshot five months earlier. In exchange for volunteering for
this experimental procedure, he was granted amnesty.
Specifically, retaining their central connections to
the spinal cord, the 8th and 10th intercostal nerves
were freed laterally and sectioned. The nerves were routed through the
spinal canal and connected to either 1) the distal ends of severed sacral
nerve roots or 2) implanted into the conus medullaris.
Although the patient related new phenomena in his
legs and bladder to the procedure, he died four months later. After
autopsy, histological analysis indicated the continuity of intercostal
nerve axons into both the sacral roots and spinal cord.

11) Dr.
A. Chiasserini (France) even earlier
in time, connected functional intercostal to cauda equine nerves in four
males with paraplegia from traumatic injury. Patient age
ranged from 21-29, and the time since injury from 1-15 months.
Procedurally, two intercostal nerves (again, nerves that lead from the
spinal cord around each rib to the sternum) were dissected out and
connected to cauda equine nerve roots (the roots descending from the lower
cord). Although one patient died postoperatively of pulmonary edema, the
other three all regained bladder function.

12) Drs.
Charles Frazier & Charles Mills
(USA): Although we tend to think of the creation of new
function-restoring, neuronal connections at the forefront of knowledge,
amazingly, such connections were used nearly a century ago to restore
bladder function in a 27-year-old man.  The patient sustained a L2 injury when a gas tank exploded near him.
Although he regained some function over time, his “bladder continued
paralyzed,” and he had “absolute incontinence.”
The patient sustained a L2 injury when a gas tank exploded near him.
Although he regained some function over time, his “bladder continued
paralyzed,” and he had “absolute incontinence.”
Again, a functional neuronal connection was made from
above the injury site to paralyzed nerve roots below the injury site.
Specifically, eight months after injury, the patient under went surgery in
which a functional L1 nerve above the injury site 1) “was divided
extradurally at its exit from the spinal canal and brought within the
dural sac” and 2) then sutured end-to-end to S3 and S4 nerve roots. Eight
months after the operation, the patient regained some bladder control.

13) Dr.
Basil Kilvington (Australia):
In 1906, Kilvington attempted to restore bladder function in three dogs
connecting sacral and lumbar nerve roots (Br Med J April 27, 1907).
Based on these animal experiments, which had marginal results, as well as
research using cadavers, he attempted this crossover surgery in a
40-year-old man, who six years previously had sustained a T11-12 injury
after falling from a tree. Ten days after performing a laminectomy on a
patient, Kilvington went in again to connect the nerve roots;
unfortunately due to the dense scar formation accruing from the initial
surgery, he was unable to continue.
TOP