1) Diapulse Electromagnetic
Therapy
2) Oscillating Field Stimulation
3)
Repetitive Transcranial Magnetic Stimulation
4)
Magnetic Molecular Energizer (MME) Therapy
1) Diapulse
electromagnetic therapy: Diapulse is a device that directs a
pulsed-electromagnetic field (PEMF) to an area of injury. Animal and human
studies indicate that this treatment soon after SCI protects neurons,
promotes regeneration, and minimizes lost function. In addition, Diapulse
greatly accelerates the healing of SCI-associated pressure sores.
Description: Diapulse directs
electromagnetic energy to a specific body area via a cylindrical treatment
head mounted on an adjustable bracket. 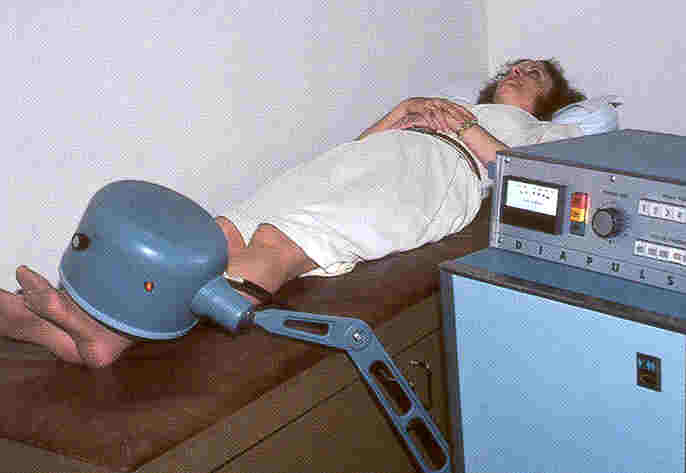 Because
the device pulses its electromagnetic output, it emits energy for only a
fraction of time, allowing any heat associated with the transferred energy
to dissipate. Diapulse’s electromagnetic output is often pulsed at 600
pulses per second with each pulse lasting 65 microseconds (1 second = 1
million microseconds). Hence, this pulse rate corresponds to the device
being off 25 times longer than it is on.
Because
the device pulses its electromagnetic output, it emits energy for only a
fraction of time, allowing any heat associated with the transferred energy
to dissipate. Diapulse’s electromagnetic output is often pulsed at 600
pulses per second with each pulse lasting 65 microseconds (1 second = 1
million microseconds). Hence, this pulse rate corresponds to the device
being off 25 times longer than it is on.
History: The Diapulse prototype was
developed in the early 1930s by physician Abraham Ginsberg and physicist
Arthur Milinowski, who reported their initial clinical experience and
animal research with the device to the 1934 & 1940 New York Academy of
Medicine. Because the technology behind the device was used to develop
radar, the device’s emergence as a healing modality was delayed due to
World War II security concerns. Research was resumed in the 1950s by the
US military, which after extensive studies concluded that the device was
safe and effective. About this time, the driving force behind Diapulse
shifted from Ginsburg to Dr. Jesse Ross, a biophysicist who created the
Diapulse Corporation of America (Great Neck, NY) and launched ambitious
research with universities and clinicians around the world.
Diapulse Research
Numerous studies
support Diapulse’s potential to treat neurologically associated problems
and exert neuroprotective and -regenerative influences. After
nervous-system injury, Diapulse helps to restore the membrane potential
(concentration difference of charged solutes between the cell’s inside and
outside) necessary to ensure cell survival and to enhance
recovery-promoting blood flow.
Blood Flow: Dr. W. Erdman
(Philadelphia, Pennsylvania) demonstrated that Diapulse increases systemic
blood flow without elevating pulse rate or blood pressure (Am J Ortho
2, 1960). This effect is most likely due to the ability of
Diapulse-generated fields to induce cells to align in a pearl-chain
fashion. When the device was turned off, the cells reassumed a random
distribution. With such a pearl-chain alignment, blood cells can more
efficiently pass through a given vascular space, like cars traveling in
the same direction on parallel lanes instead of “bumper” cars.
As in all injuries, blood flow affects recovery after
SCI. Specifically, the injury to the cord compromises blood flow, which,
as a consequence, aggravates neurological damage. Given Diapulse’s ability
to enhance blood flow, it is not surprising that the device promotes
healing after SCI.
Supporting Animal Studies : Drs. D.
Wilson and P. Jagadeesh (Leeds, UK) examined the effects of Diapulse
therapy on cats whose spinal cords were half cut (hemicordotomy) (Paraplegia
14, 1976). Three months after hemicordotomy, compared to controls,
Diapulse improved functional recovery, reduced scar formation and
adhesions, increased the number of axons transversing the injury site, and
promoted the integration of peripheral nerve grafts that had been inserted
to bridge the lesion.
Because surgeons are beginning to use peripheral
nerve tissue to bridge spinal cord lesions in human, Diapulse’s ability to
accelerate regeneration in peripheral tissue also has important
therapeutic implications for SCI.
Dr. Wise Young (New York, New York) showed that
Diapulse reduces calcium at the injury site in cats injured through
impact. Because calcium causes secondary neuronal cell death, this
Diapulse-induced reduction lessened neurological damage and, in turn,
preserved function. Specifically, Young reported that 1) the majority of
Diapulse-treated cats were walking four months after surgery compared to
none in the control group and 2) that the device was superior to treatment
with the steroid methylprednisolone, now considered a post-injury
treatment standard due ironically to Young’s efforts (Presentations
at1983 & 1984 Meetings of American Paralysis Association and 1984 Meeting
of the Society of Neurological Surgeons).
SCI Human Studies: Dr. M Weiss et al
(Warsaw, Poland) carried out a promising SCI study in 1980. Acutely
injured patients were picked up by helicopter and brought to Warsaw where
they were treated with Diapulse. Of the 97 treated patients, 38 had
pronounced neurological improvement; of these, 28 had substantial
functional gains, and 18 were discharged with only slight impairment of
the extremities (Narz Ortoped, Pol 45(3) 1980). Unfortunately,
because Weiss died soon after publishing these initial results, combined
with post-communism social upheaval, this promising research was not
continued.
Dr. W. Ellis anecdotally noted that PEMF given for
pain in patients with chronic SCI resulted in sensory or motor improvement
in seven of 13 patients (Bioelectromagnetics 8(2) 1987). Ellis
hypothesized that these fields can normalize viable but dysfunctional
neuronal structures.
Finally, Diapulse therapy was recently used in
conjunction with a function-restoring surgery in which olfactory tissue
was transplanted into the SCI injury site (Lisbon, Portugal).
Specifically, two Americans with quadriplegia were treated with Diapulse
several days before and after surgery to promote neuronal regeneration
(private communication). Although it is difficult to sort out the relative
contributions of the surgery, post-surgical rehabilitation, or Diapulse
therapy, one of the patients had so much functional recovery that she was
featured on a PBS documentary.
Pressure Sores: A number of studies
demonstrate that Diapulse treatment greatly accelerates the healing of
pressure sores, a serious SCI-associated problem. In a specific
SCI-focused, double-blind study, Dr. C. A. Salzberg et al (Valhalla, NY)
showed that the pressure sores of Diapulse-treated patients with SCI
healed on average in 13 compared to 31.5 days for controls (Wounds
7(1), 1995).
The following case study, reported in
PN/Paraplegia News (September 2003), is indicative of Diapulse’s
potential for treating SCI-related pressure sores:

2) Oscillating Field Stimulation
for Acute SCI:
Evidence indicates that oscillating field stimulation (OFS) minimizes
neurological damage after acute SCI. The therapy has been developed by Dr.
Richard Borgens and colleagues at Purdue University (Indiana, U.S.).
OFS therapy is
based upon numerous observations that appropriate electrical cues guide
and promote neuronal growth. For example, studies suggest that in early
development, naturally occurring voltage gradients channel nascent neurons
down the neural tube, the spinal cord’s anatomical precursor. In addition,
studies indicate that 1) regenerating axons are attracted to an electric
field cathode (i.e., negative pole of applied field) and 2) such a field
may alter glial cell density and organization within the injury scar in a
fashion that is less inhibitory to regeneration.
Because implanting
the cathode above or below the SCI injury site will promote neuronal
growth only in one direction through the injury site, Borgens et al
developed the OFS device in which polarity is alternated every 15 minutes.
With such a device, regeneration in both ascending and descending neurons
is stimulated.
OFS therapy is only
beneficial for acute injury. If device implantation is delayed several
months, regenerative benefits will not accrue.
Animal Research:
The OFS approach is based on extensive research by Borgens and colleagues
using dogs with naturally occurring SCI, often due to explosive disk
herniation that rapidly progresses into complete SCI. This research
includes two randomized, controlled trials in dogs (Borgens et al. J
Restorative Neurol Neurosci 5, 1993 and Borgens et al. J
Neurotrauma 16 1999), which laid the foundation for the recent trial
in humans discussed below.
In Borgens’ 1999
dog study, OSF devices were implanted in 20 paraplegic dogs and compared
to 14 dogs with an implanted sham device. After six months, overall
improvement, measured by a variety of neurological assessments, was
greater in OFS-treated dogs.
Human Study:
Given the results of these dog studies, the Food and Drug Administration
(FDA) approved a Phase-1 clinical trial to assess safety in 10 patients
with complete injuries ranging from the C5 to T10 level (J Neurosurg
Spine 2, 2005). The patients age ranged from 18 to 43 (median age 23)
and all but one were males. Six injuries were due to motor or all-terrain
vehicle accidents, two from falls, one from diving, and one from violence.
Within 18 days of
injury, the cylindrical OFS device (11-cm long; 1-cm diameter) was
implanted in the patient’s paraspinous musculature below the injury site
to minimize pain or discomfort. Emanating from the device are two sets of
three electrodes. The electrodes in one set are sutured to the spine’s
spinous process and right and left facet joints one segment above the
injury site, and the other leads are connected to the same points a
segment below the injury site. For example, for a C6 injury, the
electrodes would be connected at the C5 and C7 level. The OFS device was
removed at 15 weeks.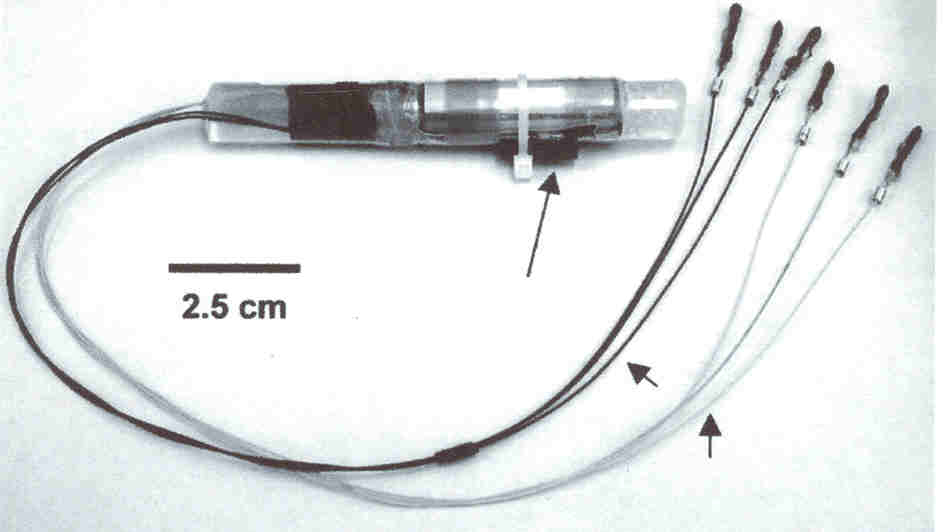
Study results
indicated that the procedure was safe, the goal of this Phase-1 trial.
A variety of
functional assessments were carried out at six months and one year after
implantation. All subjects demonstrated improved sensation, and some
regained significant motor or sexual function.
However, because no
controls were included in this study designed to assess safety, overall
efficacy could not be directly evaluated as in the case of the
aforementioned dog studies. Although improvements were noted for most
subjects a year after implantation, some recovery is routinely noted
during this post-injury period. As such, since the study had no built-in
reference point, results were compared to patient improvements documented
in the third NASCIS (National Acute Spinal Cord Injury Study) trial. This
comparison strongly suggests that OFS therapy exerts beneficial effects
after acute injury in humans.
The FDA has
improved additional testing in patients with acute SCI, and Purdue
University has licensed the technology to Andara Life Sciences, Inc.
Recently reported animal research has suggested that in addition to its
primary role, the OFS device may be an useful platform for delivering
various factors to the injury site that enhance neuronal growth, such as
inosine.

3)
Repetitive Transcranial Magnetic Stimulation
(rTMS) generates a pulsed electromagnetic field about the strength of an
MRI scan. By placing the device close to the scalp, it stimulates the
brain’s cerebral cortex, in turn activating descending neuronal pathways.
Traditionally, rTMS has been used to treat depression and other
psychiatric disorders.
Poirrier et al (University of Liege, Belgium) showed
that rTMS promotes restoration of locomotion in rats with acute,
incomplete injuries, especially lower thoracic injuries. The investigators
speculated that in such low injuries, rTMS therapy activates the spinal
cord’s ambulation-promoting central pattern generator.
Dr. Davey and colleagues at United Kingdom’s Charring
Cross Hospital and Stoke Mandeville Hospital have treated four individuals
with chronic, incomplete injuries with rTMS. The investigation was based
on the belief that rTMS weakens intracortical inhibition and thereby
enhances cortical drive to surviving corticospinal neurons, i.e., it
easier for the brain’s signals to reach the body.
Of the four subjects, three were men (age 41, 54, &
54; 7-8 years post injury) and one was female (age 26; 15 months post
injury). All had sustained C5-incomplete injuries as determined by ASIA
criteria (American Spinal Injury Association)
These subjects were initially treated an hour daily
for five days with a sham treatment (consisting of occipital cortex
stimulation) and then for the same period with the therapeutic treatment
(motor cortex stimulation). Various electrophysiological, clinical, and
functional measurements were carried out before, during, and after
treatments.
Results indicated no difference between baseline
functioning and after sham treatment. However, the therapeutic treatment
(i.e., over motor cortex) resulted in a 38% drop in intracortical
inhibition as measured electrophysiological assessments. This reduction
was accompanied by both motor and sensory improvements as evaluated by
perception of skin electrical stimulation, ASIA scores, and the time
required to complete a peg-board test. The treatment-associated benefits
lasted for several weeks.
Drs. Karen Bunday and Monica Perez (USA) used a
combination of precisely sequenced transcranial magnetic stimulation and
electrical stimulation of the ulnar nerve in the wrist in an effort to
enhance hand function in 19 individuals with chronic, incomplete
cervical injuries ranging from the C4-C8 level. Age averaged 48 years,
and all but two were men. All had been injured for at least one year and
had residual sensory and motor hand and arm motor function. When the
stimulation from the two devices was exactly timed, temporary
improvements were noted in hand-muscle strength and the ability to grasp
and move small pegs. The improvements lasted up to 80 minutes.

4)
Magnetic Molecular Energizer (MME)
has been
used to treat a variety of ailments in pilot studies, including neurological disorders
such as SC I,
head injury, multiple sclerosis, stroke, cerebral palsy, Parkinson’s,
and Alzheimer’s disease. With MME treatment, the affected body area is
placed between two large, strong direct-current (DC) electromagnets for relatively long
periods of time.
I,
head injury, multiple sclerosis, stroke, cerebral palsy, Parkinson’s,
and Alzheimer’s disease. With MME treatment, the affected body area is
placed between two large, strong direct-current (DC) electromagnets for relatively long
periods of time.
The MME device generates
a powerful 3,000-5,000 gauss, DC magnetic field. For comparison sake,
the Earth’s magnetic field is about 0.5 gauss, a refrigerator magnet is
about 10 gauss, and commonly used MRIs, can exceed 10,000 gauss. To
accrue benefit, patients often spend hundreds of hours exposed to
MME-generated electromagnetic fields. Because of the time required,
sessions are frequently scheduled on consecutive days, usually at nights
when patients can sleep.
Although few results have been reported for SCI, a
pilot study looked at the effectiveness of treating 12 individuals with
multiple sclerosis with MME therapy. Ten had “marked improvement.”
As discussed elsewhere in this report, scientists
have shown that electromagnetic fields influence the expression of
neuronal stem cells, and, as such, may be useful in enhancing the
effectiveness of the many SCI-related stem-cell transplantation programs
emerging throughout the world. Consistent with these findings, studies
suggest that
MME-generated electromagnetic fields will do so also. As a result of
these findings, several patients have combined MME treatment with
stem-cell transplantation.
According to Dr. Dean Bonlie, the developer of the
MME device, over 30 patients with SCI have been treated with
MME-generated electromagnetic fields at five clinics in the US and
Canada. According to Bonlie, “some have had astounding success and some
less than astounding.” Of the 16 patients he has treated, only two
didn’t have some improvement. Although most of the treated patients had
more long-term injuries, he believes MME therapy will be most effective
if initiated sooner after injury. For example, a patient he treated five
weeks after injury had some of the most dramatic improvement.
Bonlie believes that simultaneous treatment with
human growth hormone will enhance MME treatment effectiveness. In his
case, he has used a homeopathic preparation of human growth hormone (see
discussion of homeopathy later in this report) and since doing so, has
noted more improvements in MME-treated patients. He also believes that
MME treatment would be most effective if the injury-site scar can be
removed before treatment to enhance regenerative processes - a procedure
that has been done in some stem-cell transplantation programs.
In one anecdotal case discussed on a SCI-discussion
forum, a 23-year-old male reported regaining significant function after
multiple MME sessions. He started MME therapy 5.5 months after
sustaining an incomplete cervical C6 injury from a motorcycle accident.
Cumulatively, he received ~1,800 hours of treatment, often 16-18 hours
daily, much of it while he was sleeping. The MME-directed
electromagnetic field covered an area from his forehead to his nipples.
He also traveled to China for umbilical stem-cell
therapy and started more aggressive physical rehabilitation. As a result
of his cumulative efforts, he recovered a variety of functions,
including more hand and triceps control, abdominal and back function,
and sensation in his groin and hip region.
TOP